7 Key Parts (7 Ts) of The Massive Hemorrhage Protocol
Overview
Massive Hemorrhage Protocol (MHP) is a structured approach to managing
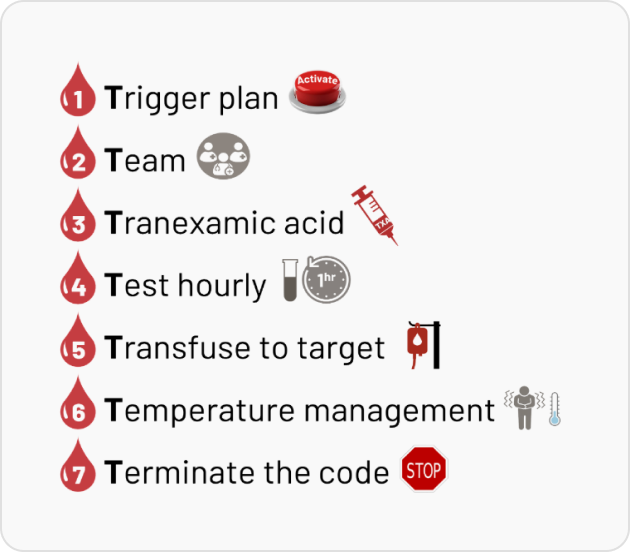
severe bleeding by ensuring timely activation, coordinated teamwork, and evidence-based interventions. This module outlines the “7 Ts” of MHP: Trigger, Team, Tranexamic Acid, Testing, Transfusion, Temperature, and Termination aimed at optimizing resuscitation strategies and minimizing complications, as per latest evidence and best practices.
Top Questions
- 1. What is the Trigger Plan in an MHP?
- 2. What should be the structure of an MHP Team?
- 3. What is the role of Tranexamic Acid in managing MHP?
- 4. Why should the patient be Tested Hourly during an MHP?
- 5. What does the principle of 'Transfuse to Target' mean during an MHP?
- 6. What is the role of Temperature in managing an MHP?
- 7. What is meant by 'Terminate The Code' during an MHP?
- 8. References
- 9. The Massive Hemorrhage Protocol Quiz
Recommended Modules
Warfarin Reversal
Effective warfarin reversal is a critical aspect of bleeding management, requiring timely and evidence-based decision-making. This module answers key clinical…
Enroll NowDOAC Reversal
Managing bleeding in patients on Direct Oral Anticoagulants (DOACs) requires a rapid and informed approach. This module provides guidance on…
Enroll NowPCC and FC in Cardiac Surgery
This module focuses on the role of Prothrombin Complex Concentrates (PCC) and Fibrinogen Concentrate (FC) in managing bleeding during cardiac…
Enroll Nowtreatthebleed©2025



